
Acella, the makers of NP Thyroid, issued a voluntary recall notice on May 22, 2020.
This is part 1 of a two-part series reviewing the drug recall notice’s statements and the science behind them.
In this post, I put this recall in the context of pharmaceutical guidelines and manufacturing challenges. I also portray the variable responses a patient could have to a slight increase in the tablet’s potency.
First of all, we should applaud Acella for being so conscientious about the exact amounts of liothyronine (LT3) hormone in their product. They tested, they were honest, and they dutifully issued this voluntary recall. The FDA is sharing Acella’s announcement — Yes, they are being “proactive,” as they state. It’s good for the reputation of desiccated thyroid pharmaceuticals to be conscientious and to prove that you’re being watchful. It shows that you’re worthy of trust.
However, the statement mathematically exaggerates the total magnitude of the pharmaceutical error per tablet. By focusing only on the potency error in the T3 content, it does not remind readers each tablet contains far more T4 hormone than T3 hormone, and the 115% superpotency of the T3 portion constitutes only 102% of the total.
Next, we should put this recall in context of other drug recalls. These kinds of quality control issues happen now and then to all drug categories, and thyroid pharmaceuticals of all types, not just desiccated thyroid, face them. A synthetic Levothyroxine recall in January 2020 in the UK is similar in nature and degree.
Finally, and most importantly, the recall statement also claims that an increase in T3 potency alone, of this magnitude, may cause “signs and symptoms of hyperthyroidism (overactive thyroid).”
The risk of hyperthyroid symptoms is overblown, and it is not the only direction of risk. In some individuals, the extra T3 could have a rebound effect toward symptoms of tissue HYPOthyroidism, as I explain. In others, adding T3 could help, or could make no difference. Only those who are already near mild overdose would be at risk of this particular adverse effect.
Human response to this pharmaceutical error will be highly variable from patient to patient, given the complexity, individuality, and flexibility of thyroid hormone metabolism.
In Part 2 of the series, in a separate post, I will focus on a more complex and controversial topic, their cautions regarding risk of adverse maternal health outcomes.
Acella’s statement

The statement that Acella made on May 22, 2020, which FDA published on their website, is as follows:
“Acella Pharmaceuticals, LLC is voluntarily recalling a total of 13 lots of 30-mg, 60-mg and 90-mg NP Thyroid® (thyroid tablets, USP) to the consumer level. The products are being recalled because our testing has found these lots to be superpotent. The product may have up to 115.0% of the labeled amount of Liothyronine (T3).
Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive superpotent NP Thyroid, may experience signs and symptoms of hyperthyroidism (overactive thyroid) which include, but are not limited to, weight loss, heat intolerance, fatigue, muscle weakness, hypertension, chest pain, rapid heart rate, or heart rhythm disturbances. Pregnant women who take superpotent NP Thyroid may also experience negative maternal and fetal outcomes including miscarriage and/or impairment to fetal development.”
“Patients should talk to their healthcare professional before they stop taking their NP Thyroid medicine. To date, Acella has received two reports of adverse events known to be related to this recall.
NP Thyroid (thyroid tablets, USP) is composed of levothyroxine and liothyronine, and used to treat hypothyroidism (underactive thyroid).”
… “Acella is proactively notifying its wholesalers by email and phone to discontinue distribution of the product being recalled and is arranging for return of all recalled products.”
“Patients who are currently taking NP Thyroid from the lots being recalled should not discontinue use without contacting their healthcare provider for further guidance and/or a replacement prescription.”
“This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.”
Acella pharmaceuticals
The recall notice also includes information about the lot numbers and labels of the medication, and it provides contact information to report any “Adverse reactions or quality problems experienced with the use of this product.”
They are being cautious and apologetic.
The error that caused the recall
The reason for Acella’s voluntary recall was that it exceeded the FDA standard tolerance for the stated potency one of its two ingredients, liothyronine (LT3), by 115%.
The FDA allowance for potency in this general category of medication is 90 to 100% of the stated dose, so it was 5% above the specification limit.
Essentially, each 60 mg of NP Thyroid was supposed to provide 38 mcg T4 + 9 mcg T3. The recalled lots provided 38 mcg T4 + 10.35 mcg T3 per tablet.
The excess above the labelled amount was an extra 1.035 mcg T3.
The FDA manufacturing standard is 90-110% potency for DTE.
The vast majority of pharmaceuticals are held to a potency standard of 90-110% of the active ingredient stated on the label.
This is the quality standard that applies to NP Thyroid and other DTE brands, as well as for other drugs.
Interestingly, synthetic levothyroxine is held to even higher standards, as a drug designated to have a “narrow therapeutic index.” The specifications were updated from 90-110% to a “narrowed 95–105% potency acceptance range for L-T4 tablets in the 2006 update to the FDA Guidance” (Eisenberg and Distefano, 2009).
This stricter guidance in 2006 applied to synthetic LT4 medication did not apply to synthetic LT3, nor to the category of animal-derived desiccated thyroid pharmaceuticals such as NP Thyroid, which were still held to the widespread standard of 90-110%.
Total potency error per tablet = 102%
Given the tablet’s two active ingredients, this is in fact an error of 102% superpotency per tablet containing T4 + T3, even though it had 115% of the smaller ingredient, T3.
The tablet’s main therapeutic ingredient is porcine-derived levothyroxine hormone, LT4. The product information for NP Thyroid states clearly that it contains “38 mcg levothyroxine (T4) and 9 mcg liothyronine (T3).”
Here is how one could calculate the total “superpotency” per tablet :
- The relative potency of the 38 mcg LT4 was not mentioned in the recall, so let’s presume for this calculation it was accurate at 100%.
- The potency of its 9 mcg LT3 was on average 115% of the stated dose. Take 9 and multiply it by 1.15, and you learn it contained about 1.035 mcg too much LT3.
- Each 60 mg tablet contains 38mcg+9 mcg of active hormone, a total of 47 mcg of active pharmaceutical ingredient per 60 mg tablet.
- Add approx. 1 micgrogram of T3 to the total. You will get 48 mcg instead of 47 mcg.
Therefore, each tablet had 1.02% “superpotency” in micrograms of active ingredient.
How common are stability recalls?
This error, both in type and degree, should be read in the context of other drug recalls of this nature.
The recall was likely due to factors interfering with stability after the product was packaged, not quality control at an earlier phase such as in the mixing of raw ingredients. The recall involved lots of medication already in packaging.
Buehler and Hyunh-Ba explain that potency of many drugs can be influenced by “storage conditions,” “pH, light, temperature, and humidity,” and “interactions with excipients and packing materials.”
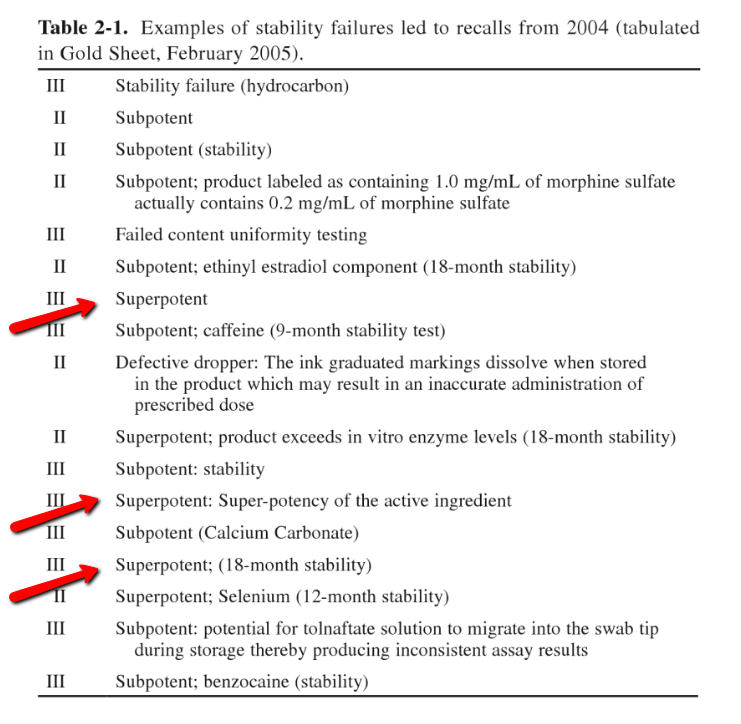
The 2009 textbook Pharmaceutical Stability Testing to Support Global Markets includes a table describing stability reasons for all recalls in 2004.
The arrows indiate that the Acella NP Thyroid recall fits within 3 of the 17 reasons for a product recall, since the table had redundancies within it.
All thyroid pharma stability is challenging
Thyroid hormone pharmaceuticals are well known to be very vulnerable to changes in storage conditions.
For example, a recent pharmacy sciences doctoral thesis (Shaban, 2018) discovered that an increase in storage temperature over 40 degrees Celsius resulted in a percentage of levothyroxine (LT4) being converted to its metabolites Triac, Tetrac, and T2. This “clearly indicates that levothyroxine is chemically unstable at high temperature.”
Eisenberg and Distefano, 2009, noted more broadly that “L-T4 tablet stability is known to be sensitive to factors such as light, temperature, and moisture.”
In fact, as cited by Lowe (2009), the historical FDA recall record for synthetic levothyroxine was significant: “Between 1990 and 1997: 10 recalls, 150 lots, and 100 million tablets.”
Referring to Duffy’s 2006 FDA slide presentation, Lowe’s article pointed out that historically, prior to stricter regulation, many levothyroxine manufacturers intentionally made pills super-potent to compensate for this instability problem:
until the FDA stopped the practice, many companies engaged in “stability overage”; that is, the companies would add more than 100% of the T4 designated on the product label. They did so because they assumed that potency would be lost, and they compensated for the loss by packing extra T4 into the tablets.
(Lowe, 2009)
Overall, as Lowe states, “the stability of levothyroxine has been far more in question at the FDA than has that of desiccated thyroid.” This is likely because LT4 is now the more commonly prescribed thyroid hormone preparation and has been the focus of intense FDA scrutiny since late 1990s.
Wockhardt LT4 recalled for having excess LT3 content
A recall of a different synthetic LT4 preparation in the UK, a liquid formulation, was announced in January 2020 for a similar reason: Liothyronine (LT3) is considered an “impurity” when the drug is not supposed to have more than 2%.

The tablet was not supposed to contain any liothyronine (LT3) at all, yet specifications permitted it to contain a small amount anyway, because LT4 inevitably degrades on the shelf.
The Wockhardt recall explains that “Liothyronine is a breakdown product that is present in Levothyroxine formulations.”
How much LT3 did Wockhardt’s LT4 contain by mistake? The recall statement does not say, which is worrisome. NP Thyroid was ethical to specify 115%. Wockhardt kept it a secret. Perhaps Wockhardt wished to avoid the public shame and panic of admitting it exceeded the limit by a large amount.
What are the specification limits for LT3 in LT4-only preparations?
I searched extensively but could not find any UK specification limits for levothyroxine impurities. However, UK pharma regulation tends to harmonize with the US. I found an October 1, 2019 Revision Bulletin (PDF) with specifications from the USP (United States Pharmacopeia). For each tablet :
- Levothyroxine Sodium Tablets contain NLT [no less than] 95.0% and NMT [no more than] 105.0% of the labeled amount of levothyroxine sodium.
- Impurities — Limit of Liothyronine Sodium. … Acceptance criteria: NMT [no more than] 2.0% of liothyronine sodium
To compare this amount to the relative dose of NP Thyroid,
- NP Thyroid 60 mg contained 38 LT4 + 9 LT3 = + 1.035 mcg extra LT3
- Wockhardt, for every 38 mcg LT4 + 2% of LT3 = + >0.76 mcg extra LT3
However, for all we know, the Wockhardt product may have contained more than 1 mcg for every 38 mcg of LT4, or more than 2 mcg above the allowance for every 100 micrograms.
I’ve now covered all the pharmaceutical contexts of this NP Thyroid recall.
Next, I’ll get into the potential human RESPONSE to “superpotent” NP Thyroid.
Continue to Page 2:
- Can a 1.053 mcg excess in LT3 “cause” thyrotoxicosis?
- Wide variation in response to DTE thyroid therapy
- Other factors that matter
- In summary: Let’s all be vigilant. Always.

